About the project
EMV Enhance (HK) Limited is a member of the Avalon Biomedical (Management) group. It was founded in 2016. We are located at the Hong Kong Science Park.
EMV Enhance is dedicated to the development and commercialization of innovative products and devices for medical use. In particular, it focuses on products and devices that are related to the prevention and the treatment of viral diseases.
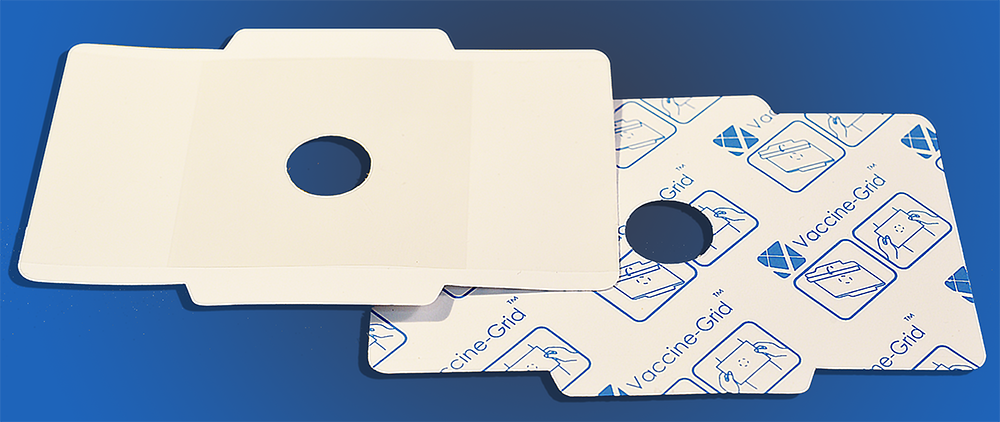
MedSpread™ and Vaccine-Grid™ are patented devices developed for enhancing the immunogenicity, i.e. the effectiveness, of intra-dermally applied vaccines. Currently, pilot productions of two products, MedSpread™ and Vaccine-Grid™ are well underway.


The Science
MedSpread™ and Vaccine-Grid™ are patented tools specially designed and developed to be used in association with Imiquimod cream.
Poor immunogenicity is well known for commercial vaccines, and particularly impacts negatively on older adults and young children. Meta-analysis estimated that the overall efficacy of these vaccines is around 70% (Osterholm et al., 2012). These problems have limited the benefits of vaccines for the elderly and young population. As these groups are at the high risks of hospitalization for influenza infection and its complications, they need the vaccine protection the most.
Attempts have been employed to improve vaccine immunogenicity. Among them, vaccination via the intradermal route (Hung 2012a; Hung2012b) and concurrent use of vaccine adjuvants aiming at recruiting the functions of the pattern recognition receptors (PRRs) in the innate immune system, including the Toll-like receptors (TLRs), have been used (Kasturi et al., 2011). Imiquimod, a synthetic Toll-like receptor 7 agonist, acts as an immune response modifier; and in a mouse model, the immunogenicity of influenza vaccine was enhanced by applying imiquimod cream. The local immune-boosting effects indicate that imiquimod can be potentially used as vaccine adjuvant to improve immunogenicity (Zhang et al., 2014). In a clinical trial in elderly subjects, pre-treatment with topical imiquimod significantly expedited, augmented and prolonged the immunogenicity of influenza vaccination relative to a vaccine-only group, with earlier and better seroconversion rate sustained to 1 year, and fewer hospitalization for influenza or pneumonia (Hung et al., 2014). In a phase 2b/3 trial, topical application of imiquimod before intradermal trivalent influenza vaccine significantly improved immunogenicity against the vaccine influenza strains in young healthy individuals and increased immunogenicity against the non-vaccine strains (Hung et al., 2016).
However, performing such pre-treatment along with vaccination is inconvenient in clinical settings and compromises adoption of the process. This is particularly true in mass vaccination programs. Furthermore, additional benefit can be realized by maintaining the pre-treatment imiquimod cream at or near the injection site for a prolonged period of time following vaccination.
In order to provide a safe and convenient method to enhance the effectiveness of intradermal vaccinations, we have developed the MedSpread™ and Vaccine-Grid™.
MedSpread™ provides a convenient way to measure and deliver an exact amount of imiquimod cream to the vaccination site intended for intradermal injection. It also assists in the measurement of and spreading the imiquimod cream on the vaccinee’s skin to a 4 cm x 4 cm area.
Vaccine-Grid™ is subsequently applied to the area where imiquimod cream has been applied. After the imiquimod cream has been removed from the central hole of the Vaccine-Grid, the vaccinee’s skin is cleaned and disinfected by an alcohol swab; and then the vaccine is injected intradermally. After vaccination, the Vaccine-Grid™ is left attached to the vaccinee’s skin for 6 – 8 hours before removal.
Reference
- Hung IF, Levin Y, To KK, Chan KH, Zhang AJ, Li P, Li C, Xu T, Wong TY, Yuen KY. 2012a. Dose sparing intradermal trivalent (2010/2011) vaccination overcomes reduced immunogenicity of the 2009 H1N1 strain. Vaccine 30:6427-35.
- Hung IF, Levin, To KK. 2012b. Quantitative and qualitative analysis of antibody response after dose sparing intradermal 2009 H1N1 vaccination. Vaccine 30:2707-08.
- Hung IF, Zhang AJ, To KK, Chan JF, Li C, Zhu HS, Li P, Li C, Chan TC, Cheng VC, Chan KH,Yuen KY. 2014. Immunogenicity of intradermal trivalent influenza vaccine with topical withtopical imiquimod: a double blind randomized controlled trial. Clinical Infection Diseases 59(9):1246-55.
- Hung IF, Zhang AJ, To KK, Chan JF, Li P, Wong TL, Zhang R, Chan TC, Chan BC, Wai HH, Chan LW, Fong HP, Hui RK, Kong KL, Leung AC, Ngan AH, Tsang LW, Yeung AP, Yiu GC, Yung W, Lau JY, Chen H, Chan KH, Yuen KK. 2016. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial. Lancet Infectious Diseases 16(2):209-18.
- Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B, 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543-47.
- Osterholm MT, Kekkey NS, Sommer A, Belongia EA. 2012. Efficacy and effectiveness of influenza vaccines: a systemic review and meta-analysis. Lancet Infectious Diseases 12:36-44.
- Zhang AJ, Li C, To KK, Zhu HS, Lee AC, Li CG, Chan JF, Hung IF, Yuen KY. 2014. Toll-like receptor 7 agonist imiquimod in combination with influenza vaccine expedites and augmentshumoral immune responses against influenza A(H1N1)pdm09. Clinical and Vaccine Immunology 21(4):520-79.
